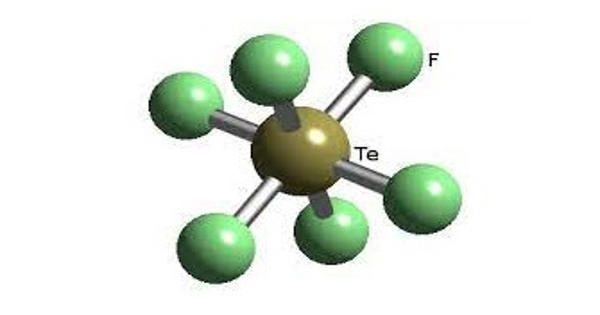
Tellurium hexafluoride is the inorganic compound of tellurium and fluorine with the chemical formula TeF6. It is a colorless and highly toxic gas with an unpleasant odor.
Preparation
Tellurium hexafluoride can be prepared by treating tellurium with fluorine gas at 150 °C. It can also be prepared by fluorination of TeO3 with bromine trifluoride. Upon heating, TeF4 disproportionates to give TeF6 and Te.
Properties
Tellurium hexafluoride is a highly symmetric octahedral molecule. Its physical properties resemble those of the hexafluorides of sulfur and selenium. It is less volatile, however, due to the increase in polarizability. At temperatures below −38 °C, tellurium hexafluoride condenses to a volatile white solid.
Reactivity
Tellurium hexafluoride is much more chemically reactive than SF6. For example, TeF6 slowly hydrolyzes to Te(OH)6:
- TeF6 6 H2O → Te(OH)6 6 HF
Treatment of tellurium hexafluoride with tetramethylammonium fluoride (Me4NF) gives, sequentially, the hepta- and octafluorides:
- TeF6 Me4NF → Me4NTeF7
- Me4NTeF7 Me4NF → (Me4N)2TeF8
Further sources
- W.C. Cooper, Tellurium, Van Nostrand Reinhold Company, New York, USA, 1971.
- K.W. Bagnall, The Chemistry of Selenium, Tellurium and Polonium, Elsevier Publishing, New York, 1966.
- R.T. Sanderson, Chemical Periodicity, Reinhold, New York, USA, 1960.
- F. A. Cotton, G. Wilkinson, C.A. Murillo, and M. Bochmann; Advanced Inorganic Chemistry, John Wiley & Sons, 1999.
- G.J. Hathaway, N.H. Proctor, Chemical Hazards of the Workplace, 5th edition, Wiley-Interscience, New Jersey, 2004.
References
External links
- Web Elements
- OSHA
- CDC – NIOSH Pocket Guide to Chemical Hazards